The cart is empty!
Local Branch News – Aberdeen Branch
The branch March meeting featured a presentation by Dr Robert Lindsay, reader in Corrosion Science and Engineering at the University of Manchester via webinar, entitled “Corrosion inhibition: Separating Fact from Fiction”. Prior to taking up this position in 2005 he had appointments at a number of other research institutions including the Fritz-Haber-Institut (Berlin), Cambridge University, and the CSIC Institute of Materials in Barcelona.
For more than a century, surface-active organic species have been employed to control the corrosion of metals/alloys. Given suitable selection, such corrosion inhibitors have proven to be highly effective, preventing significant degradation of metallic substrates even in highly aggressive environments. Corrosion inhibitors (CI) can be applied in a variety of applications, and most obviously in instances where there are large amounts of aqueous solutions such in the Oil & Gas Industry.
Corrosion inhibitors are substances that, when added in small quantities to a normally corrosive environment e.g. water, gas or oil containing, reduce the corrosion rate (CR) by bringing about a change at or near the surface, without significantly changing the concentration of corrosive species. Layers of corrosion inhibitors can be applied to surfaces to markedly reduce corrosion rate. CI efficiency is measured by comparing corrosion rates with and without the CI coating (film), a CI is considered effective if reduction in CR of >95%, and adequate for corrosion allowance of the material, is achieved.
CI can be classed as ‘2D’ or 3D’ adsorbed thin films. The presentation focused on the performance of 2D’ adsorbed films only in low pH, acidic solutions. These frequently have low persistency in operational service (unless CI replenished regularly (at least daily to the system). In an acidic environment of HCl, the main reactions on a steel substrate are the anodic reaction with iron going to its oxide and the cathodic reaction where there is a reduction of protons to form hydrogen molecules. The inhibitor needs to interfere with one or more of these reactions to be defined as an anodic, cathodic or ‘mixed’ type of inhibitor. The inhibitor forming a 2D layer on the surface, shown pictorially as either a monolayer or a bilayer, which is dynamic, forming and re-forming all the time given that there is a balanced supply of inhibitor.
For practical selection in industry, CIs are usually chosen based on users experience by empirical means (historical trial and error), or suppliers’ knowledge and or compatibility with other chemicals already deployed for different production / process reasons. However, to move on, further research is still necessary to fully understand the mechanisms, study of the adsorption thermodynamics (how strongly they bond to surface), and characterise the interfaces, which can possibly lead to ‘green’ CIs being developed.
Understanding how CIs work in detail will allow Industry to move away from an empirical selection process. i.e. are they adsorbing on a clean surface or on top/ combined with salt and oxide, surface?
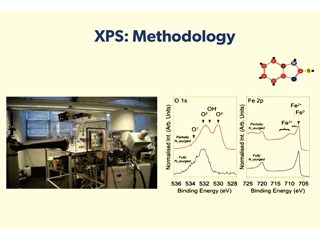
To this end, current research activity at Manchester focuses on mechanistic understanding of interface properties of relevance to corrosion. Combining electrochemical measurements with surface sensitive probes, such as X-ray photoelectron spectroscopy, to develop structure-performance relationships to enable more complete understanding of corrosion related phenomena.
Details of corrosion inhibitor-substrate interactions are being studied through detailed interface characterisation. X-ray photoelectron spectroscopy (XPS), results indicate that the chemistry of the inhibited interface is dependent on both inhibitor concentration and acid identity. The science is highly academic in nature and the physics and chemistry of the studies are best explained by watching the You Tube video presentation at: https://www.youtube.com/watch?v=_-NtzNC7BZo.
Data gathered so far has demonstrated that surface adsorption is not always sufficient for achieving the target corrosion inhibition efficiency, i.e. a surface-active agent can be bound to the surface, but not lead to sufficient reduction in corrosion rate. Moreover, XPS data can be used to argue that the widely adopted approach of determining the standard Gibbs energy of adsorption of a corrosion inhibitor from measured inhibitor efficiencies is flawed, and so should not be relied upon as a tool for corrosion inhibitor selection.
The final message from the presentation focussed on green CIs. We need inhibitors to be more sustainable and environmentally friendly, but a review of the literature shows that little progress has been made and the use of plant-based alternatives (strawberries and other examples) or recycling of waste products (such as coffee grinds) has not received much attention, or is currently judged as having little prospect. However, it should be pointed out that many research programmes have been delayed by the recent pandemic.
In April the branch welcomed Dinko Cudic, Global Technical Authority for StopaqR in a hybrid live presentation/webinar from the Jury’s Inn, Aberdeen entitled “Reducing the environmental footprint for surface preparation and coating application for onshore and offshore assets.”
Dinko is also the technical director for visco-elastic coatings, and has held senior management positions in the Sealed for Life Group who have owned the StopaqR subsidary for more than 15 years.
The energy and utilities industries are now accepting the need to find alternative technologies to reduce waste, CO2 emissions and considerable carbon footprint (product mining/transportation) for steel preparation and coating application. Dinko’s company have developed a solution that addresses, waste reduction, the lowering of CO2, noise and thermal insulation properties, not previously considered in fabric maintenance and asset integrity solutions.
Based on a compound containing non-crystalline, low-viscosity, non-crosslinked (fully amorphous), pure homopolymer polyisobutene, the presentation discussed why such technology should be considered as a viable option, in an industry that requires straightforward maintenance solutions to difficult corrosion problems both onshore and offshore.
Protective coatings are commonly applied to steels, but the integrity of these coatings is dependent on the cleanliness, profile and surface finish of the substrate, and most coatings will simply not adhere or give required lifetime performance unless there is a high-quality preparation giving an angular blast profile and no surface contamination.
In order to achieve this, degreasing compounds are often deployed, followed by abrasive blasting to prepare the new steel substrates or indeed remove old coatings to reapply new ones. The ‘media’ used for blasting is often dependent on the substrate but is essentially hard, angular grit in the form of alumina, garnet, or steel. In the blasting process there are now increasingly demands that all media and the corrosion products, and paint debris, must be safely captured and either recycled or separated and disposed of in a controlled manner, at high cost. However, the environmental cost of mining and manufacturing the media are also quite considerable and with these materials shipped around the world gives rise to a significant carbon footprint. Subsequent clean-up of the paint application equipment means that there are also waste solvents to be recycled and disposed of. Hence the present drivers towards protective coatings that can be applied without extensive surface preparation, or spray application of liquid paints.
Dinko described the target coating engineering requirements, and explained that inspiration for the development of the StopaqR compound was actually taken from chewing gum (!!), an ubiquitous material which adheres to pavements in cities around the world, which is a menace to council authorities. Incredibly, it sticks with no preparation, it flows and adheres, and seals the surface for a lifetime!
In order to develop a product for corrosion protection purposes, polymers available in the open market were studied to look at properties critical to Industry needs, and the product selected that met all criteria was polyisobutene. This product was first developed by BASF in the 1930’s and has a track record in other fields.
In the beginning, solutions were pumped and spread on to surfaces, webbing was added, and a backing or seal coat tape applied for mechanical protection, these were eventually all put into a prepared tape product that could be cut and pressed or wrapped onto surfaces. A particular characteristic of the compound developed was that it remained pliable like a putty, between the surface and the backing tape, so constantly flexible and allowing it to be pressed into all joints and profiles.
The practical application of the product in various fields e.g., buried or submerged piping, splash zone, corrosion under insulation or condensing lines will depend on the international standards and individual company codes that already exist for corrosion protection. Work originally focussed on onshore, above ground pipeline wraps in high salinity, high chloride content environments, desert environments, and then moved on to flanges and awkward junctions where the product was cut to shape and pasted onto the substrate.
The final product can be applied to flat steel in a fashion resembling ‘wallpaper’ using ~ 300mm wide rolls. Essentially the surfaces that it is to be laid onto can first be tested for adhesion by taking a sample and assessing whether it sticks or not.
For materials such as stainless steel, surface preparation would remove natural protective films so patches are generally applied directly over natural oxide or flaws. There are also specific system wraps developed with multi-layers to include corrosion protection, insulation, water impermeability, and with outer aluminium cladding.
From the original Oil & Gas industry viewpoint, StopaqR has now moved into applications in civil engineering for bridges and structural supports to offshore renewables – wind turbine towers.
One area in which the product is extremely useful, is in patching damage and sealing small areas by simply cutting patches like plasters from larger sheets and and then applying them.
The presentation also considered the cost advantages of using StopaqR low carbon footprint products. For high quality paint systems 80% of the cost of application is in surface preparation and application but that cost can drop by about 20% for StopaqR type protective compound. The cost over time and the cost to the environment are also significantly reduced along with reduction in risks & HSE associated with the surface prep, materials handling and disposal.
A recent case study of actual cost for the application of North Sea approved epoxy paint systems versus StopaqR for a fabric maintenance area of 550m2 was described. The data from these trials in respect of materials, application and clean up was fully evaluated. Costs for StopaqR system were found to be ~ 2/3 that of a conventional paint system, excluding lifetime costs which can extend maintenance
intervals by 3x.
A comprehensive Q&A took place following both the above presentations which featured significant international attendance.
The branch intends to hold its AGM in September 2022, ahead of the start of the new technical sessions. Abstracts of potential papers for its technical programme are always welcome and these should be sent to:
Hooman Takhtechian, htakhtechian@oceaneering.com
![]()
Figure 1 – The Experimental Toolbox
![]()
Figure 2 – Summary of Testing Techniques Deployed.
![]()
Figure 4 – Green CI Definition.
![]()
Figure 5 – StopaqR compound product in-service applications.