Rewards, Awards, and Benefits of Participating in the Young Engineer Programme
Here’s a case study for all young corrosion engineers:
Do what you love to do. Receive grade A mentorship in the process. Develop your learning and understanding, and make new connections to deepen your professional network. Oh, and win a fully expensed trip to the 5-day AMPP Annual Conference & Expo in the USA.
Too good to be true? Not for the winning team of the 2020 cohort of the Institute of Corrosion’s Young Engineer Programme (YEP).
What is the Young Engineer Programme?
The YEP is specifically designed for engineers at the early stage of their careers in the corrosion industry. A series of lectures are presented, and the group is divided into teams and challenged to present solutions to a real-life case study.
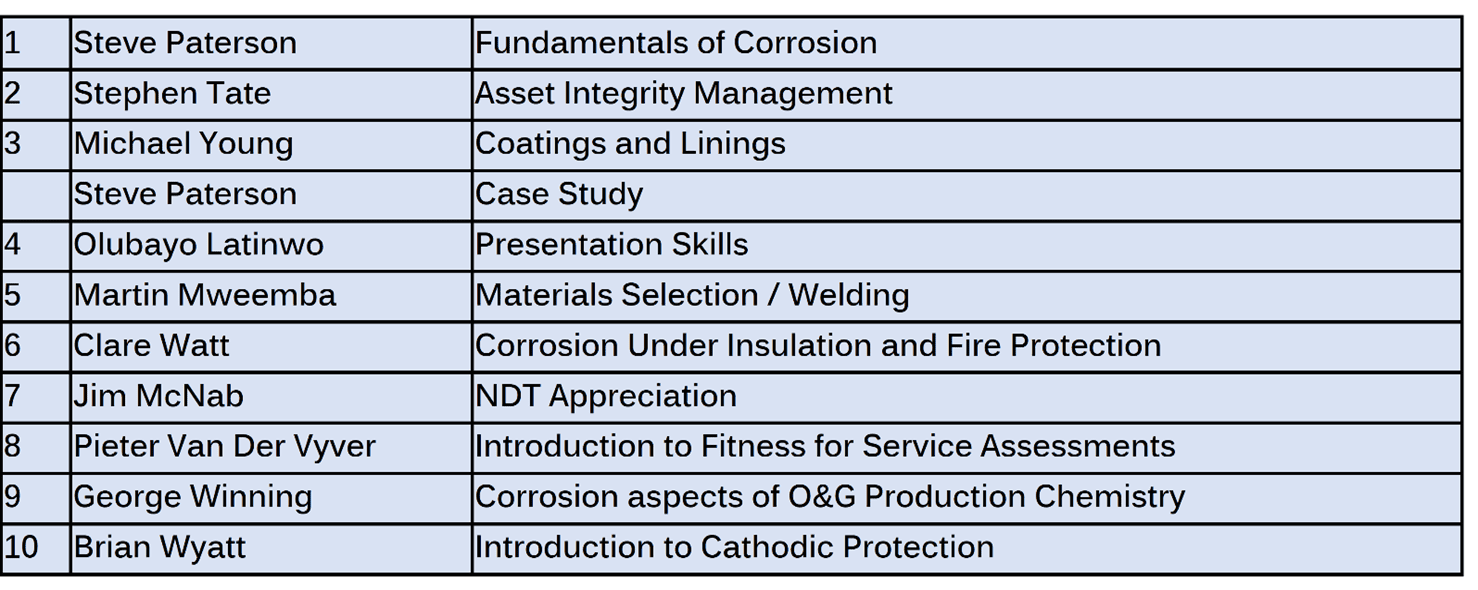
The lectures cover subject areas including:
- Basic corrosion
- Welding
- Materials
- Coatings
- Painting, fire protection and linings
- Cathodic protection
- Chemical treatments
- Presentation skills
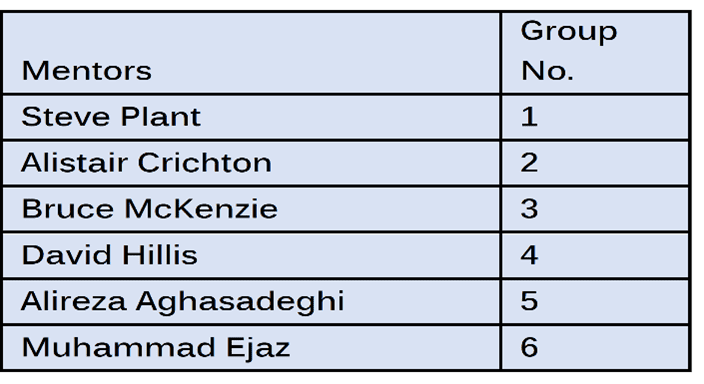
These lectures are designed to provide the theoretical and practical guidance needed to complete the case study. To aid progress toward their goal, each team is allocated a mentor with relevant and recognised industry expertise and experience.
There was a total of 30 young engineers enrolled in the 2020 cohort, all able to take advantage of this immensely valuable (and free) course. The YEP enrolled engineers divided into seven teams of between three and five for the case study.
The mentors were:
- Andrew Sturgeon, Manager Materials Engineering at Genesis Energies, London
- Chris Googan, Materials & Corrosion Engineer at Anticorrosion Engineering Limited
- Charles Barraclough, Materials and Welding Engineer
- Tasos Kostrivas PhD, ΕMBA, MSc, FIMMM
- David Shaw, Lead QC coating/insulation/PFP Saipem
- Rob Doggett, Materials and Welding Engineer at Fluor
- John Davies, Consultant QA Engineer at Fulkrum Technical Services
Throughout the programme, guests included Bill Hedges, Gareth Hinds, Steve Paterson, Danny Burkle, and Caroline Allanach and the Steering Group; Trevor Osborne, Alan Denny, Anthony Setiadi, and David Mobbs.
The case study – the challenge
The case study that the teams were asked to review and present their findings centred around a titanium pipe corrosion failure at an onshore glycol desalination plant, in which was found several leaks. The desalination plant is used to periodically remove the salts from glycol which is used for hydrate and corrosion control in gas pipelines from three offshore fields.
Particularly challenging in this case study is that the high-grade titanium spool would be expected to resist any form of corrosion in this service. After being given the complete case study, the teams were tasked to include the following in their submissions:
- Propose credible root causes for the observed defects and describe the potential failure scenarios
- Explain how you would perform a corrosion risk assessment to determine if the plant is safe to operate
- Identify what mitigation options could be applied to prolong the service life of this section of the desalination plant
- Propose alternative materials of construction for replacement pipe spools and describe the basis for the selection
- Describe what other factors should be considered in your assessment and propose possible longer-term solution(s)
The case study – presentation and judging
The teams presented their case studies to a panel of judges (Sadegh Parvizi, Chris Williams, and John Boran) on 12th November 2020. Each 20-minute presentation was followed by five minutes allotted for questioning. While no team was allowed into the presentation meeting before their time, they were permitted to remain in the meeting to hear subsequent teams present.
The presentations began at 5pm after registration and introductions. Would presenting first be best? To get your presentation completed and then relax to watch others? Or maybe presenting last would be more advantageous – with extra time to do those last-minute preparations and practice? Or would each team feel the added pressure of more eyes on them as the evening progressed?
When the final presentation had been made, the presentation session was called to a close. During a 20-minute break in proceedings, the judges deliberated, cogitated, and digested the tremendous presentations they had been served in seven courses (sorry, we couldn’t resist pinching from Lloyd Grossman’s Master Chef catchphrase!).
To be honest, there was very little to choose between the case study tasks completed. Each team’s findings had terrific merit – a testament to their mentorship, the lectures they had attended, and the collaborative capability of each team.
The deciding factor came down to presentation: the clarity and precision with which the winning team delivered its findings and answered the challenging questions posed by the judges.
And the winning team… Drum roll…
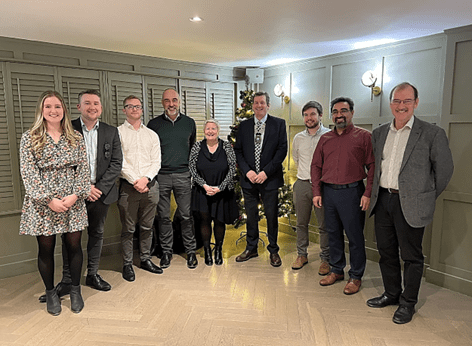
Team number four, mentored by Tasos Kostrivas, and comprising:
- Ryan Cobbs, civil engineer at Mott MacDonald
- Izabela Gajewska, corrosion engineer at Intertek
- Harry Wright, corrosion engineer at Element
- Praveena Nkumaran, mechanical engineer at Worley Parsons
- Lemoine Vincent, welding engineer at Saipem
The grand prize – the fully expensed trip to the AMPP Annual Conference & Expo 2022
Because of Covid, the delivery of the grand prize was unavoidably delayed by a year. Nevertheless, the winning team – unfortunately minus Praveena who was unable to attend – set out off for San Antonio in Texas for the AMPP Annual Conference & Expo in March 2022.
For many, this is a once-in-a-lifetime event. For only a select few young engineers, their attendance is fully expensed, courtesy of YEP sponsor BP.
This event is the largest of its kind in the corrosion world. There are more than 500 technical paper presentations, almost 500 exhibitors, opportunities to gain credits toward career development, and the chance to hear from some of the best corrosion professionals on the planet – as well as meet and connect with peers from around the globe.
And it wasn’t only these four young engineers who attended the conference and expo in San Antonio. Thanks to the sponsorship of Pipeline Induction Heat, James McGladdery (National Nuclear Laboratory) and Benjamin Lee (SGN) were selected to join the AMPP Leadership Course for their performance during the programme.
The YEP experience through the winners’ eyes
Winning at anything isn’t a cake walk. It takes hard work, effort, and determination. It takes learning and enthusiasm. How does YEP stand up to scrutiny from the inside?
Here’s what Izabela Gajewska said about her experience:
“Taking part in 2020 Young Engineer Programme was an amazing experience and a great opportunity for networking. All lectures were interesting and very educational. I got an opportunity to learn more about areas of the industry that I am not involved a lot in my daily job including welding, fire protection, and chemical treatments.
“It was a great experience to work on the case study with colleagues from different companies and industry branches. The ideas and a views of all team members were equally valuable, enhanced creativity, and were essential to solve the case study and prepare the final presentation. I enjoyed collaboration and brainstorming very much.
“During solving the case study I had to motivate myself to look through many valuable research papers and technical books recommended by my team colleagues and our mentor, Tasos Kostrivas. I had also a chance to see different approaches to solve corrosion issues thanks to the diversity of the industries in my team. Apart from this, I feel that I have improved my planning and communication skills, teamwork, did some good networking, and made new friends.
“I also was delighted to take part in the 2022 Annual Conference by NACE/AMPP (Association for Materials Protection and Performance) in San Antonio, Texas and celebrate winning the Young Engineer Programme 2020 along with my team colleagues Vincent Lemoine, Ryan Cobbs, Harry Wright.
“One of my team colleagues, Praveena Nanthakumaran was not able to attend. Fortunately, she will be able to attend the next NACE/AMPP conference in Denver next year, and celebrate her well-deserved trip to the conference in the United States.
“For me, the highlight of the NACE/AMPP conference was the EMERGing Leaders Bash which included recognition and celebration, acknowledging the accomplishments of 2022 scholarship and award recipients including my winning team and two other colleagues accepted for the NACE/AMPP Leadership Programme: James McGladdery and Ben Lee. It was an honour to be a part of this amazing and inspiring evening.”
To the present – a case study to whet the appetite
The 2022 YEP cohort have another real-life case study to become immersed in. This year’s candidates will be presenting in Aberdeen (held here for the first time, and aptly so). They have been asked to provide a corrosion risk assessment of a platform in the North Sea for a client who is planning to acquire the asset. But:
- Corrosion on the platform has been poorly managed during the past 15 years, resulting in several hydrocarbon leaks
- The teams must determine and present solutions to extend the life of the platform for another 10 years, making the exercise a real challenge
- They must also identify materials selection for a new pipeline
A challenging, real-life case study that will help all the young engineers involved improve their learning and prove their competence. A fantastic addition to any CV.
To the future – it’s time to start thinking about pre-enrolment for YEP
Demand for places in the Institute of Corrosion’s Young Engineer Programme is always high. Benefitting from lectures given by some of the industry’s brightest minds, offering the chance to network and collaborate with some of the industry’s upcoming stars, and the opportunity to be rewarded with an incredible, fully expensed experience, it is not difficult to understand why.
If you are at an early stage of your career in the corrosion industry and would welcome extra experience to set you up for the future, please contact the Institute of Corrosion. We would be pleased to answer your questions and provide details of how you may pre-enrol for our next YEP cohort.
Don’t forget, also, to follow the Institute of Corrosion on our LinkedIn page – where we post regularly to keep the corrosion community updated.
If you’re not already, why not become a member of the Institute of Corrosion? We have many different membership options, including free student membership.